Why Salt?
Taken From Out Of Harm's Way by Kenneth D. Nunn © 2011
There seems to be one question that most people have concerning long term survival, that is, salt requirements and supply. The problem comes from the lack of information available to the public. Not only the lack of information, but much of this information is confusing.
I think the first thing to do is to look at a few facts about salt. There seems to be a lot misconceptions about salt and the need for salt. Most people realize salt is something that is necessary for life. However, few understand why we require salt , how much we require, and where we get it.
First of all is the fact that sodium and sodium chloride are actually two different things, but are often used interchangeably :
• Sodium chloride : The chemical name for table salt. It is the same salt that is found in oceans and salt lakes. It also the major ingredient in commercially available table salt. The same salt that it is used as a condiment and also used in preserving food.
• Sodium : A metallic element essential for all animal life (including human) and for some plant species. Ninety percent of the sodium we consume is in the form of salt. It makes possible proper nerve conduction, the passage of various nutrients into cells, and the maintenance of blood pressure.
• Hyponatremia (salt deficiency) : Occurs when sodium levels in the blood plasma falls below acceptable levels. Symptoms include tiredness, disorientation, headache, muscle cramps, and nausea. Severe hyponatremia can lead to seizures and eventually coma.
Hyponatremia Causes :
• Result from the abnormal consumption or excretion of dietary sodium or water.
• Often occurs in patients taking diuretic drugs who maintain a low sodium diet. Diuretics which are used to correct high blood pressure by increasing the excretion of sodium into the urine. This can result in hypoatremia if too much sodium is excreted. This usually results in mild hyponatremia, but when combined with a low sodium diet or with the excessive drinking of water, severe hyponatremia can develop.
• Severe and prolonged diarrhea can also cause hyponatremia.
• Although rare, drinking excess water sometimes causes hyponatremia. This results when absorption of water into the bloodstream dilutes the sodium in the blood.
• Hyponatremia also develops from disorders in organs that control the body's regulation of sodium or water.
Death due to severe hyponatremia is rare and is usually the results of underlying conditions rather than hyponatremia.
The body is continually regulating dietary sodium. When the sodium reaches a level that is too high or too low the intestines and kidneys respond and bring the concentrations back to a normal level. The intestines are continually absorbing sodium as the kidneys excrete a nearly equal amount of sodium into the urine. If a low sodium level is detected then, the intestines increase sodium absorption, at the same time the kidneys reduces sodium release into urine.
An interesting point to bring up here is the fact that the majority of sodium chloride we ingest is excreted in urine, that is as long we are not sweating excessively. As a matter of fact, in people who remain in a steady-state in conditions of sodium and fluid balance, with little sweat loss, the amount of sodium excreted in urine is equal to the amount of intake.
The level of sodium in the blood plasma depends on the amount of sodium in relation to the amount of water in the circulatory system (arteries, veins, and capillaries). Sodium and water levels in the body are regulated by two separate systems in the body. Although separate they work together in order to regulate blood pressure when it reaches a level that is to high or to low. As stated earlier, If the sodium level becomes too low it is correct by increasing the sodium absorbed by the intestines while the kidneys reduces the amount of sodium released in urine. At the same time body water in decreased by frequent urination. The opposite would be true if the sodium level becomes too high.
Due to the fact that sodium intake is regulated by separate body functions a number of different diseases can cause hyponatremia; these include kidney diseases, pituitary gland, and hypothalamus.
Now we come to the big question how much salt should a person consume daily? There are a number of factors that will affect this, such as climate, activity and age. The information below should be looked at as a general guideline.
This information is taken from the February 2004 report from the Food and Nutrition Board. :
• Healthy 19- to 50-year-old adults should consume 3.8 grams of salt per day -- to replace the amount lost daily through sweat and to achieve a diet that provides sufficient amounts of other essential nutrients.
• The tolerable upper intake level (UL) for salt is set at 5.8 grams per day.
• Older individuals, African Americans, and people with chronic diseases including hypertension, diabetes, and kidney disease are especially sensitive to the blood pressure-raising effects of salt and should consume less than the UL.
Then you will find the following in the paper – Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate By : Institute of Medicine of he National Academies :
Human populations have demonstrated the capacity to survive at extremes of sodium intake from less than 0.2 g/day (1/3 teaspoon/day) of sodium in the Yanomamo Indians of Brazil to over 10.3 g /day (1 teaspoon/day) in Northern Japan. The ability to survive at extremely low levels of sodium intake reflects the capacity of the normal human body to conserve sodium by markedly reducing losses of sodium in the urine and sweat. Under conditions of maximal adaptation and without sweating, the minimal amount of sodium required to replace losses is estimated to be no more than 0.18 g /day (1/3 teaspoon/day).
When you consider that 1 teaspoon = 6 grams of salt and that the individual daily requirement is 3.8 grams you will see that the recommended amount of salt a person should have each day is small. Especially when you consider the natural salt content of the foods we eat and drink.
Looking at all the variables in a long term survival situation there is no way of accurately estimate the amount of salt you should cache. The information presented in this article will hopefully enable you to make an educated guess.
Even with an adequate supply of salt cached, you should make every effort to conserve your salt supply. The actual salt a person adds to their diet should be considerably less than the 3.8 grams recommended. This will ,of course, depend on the climate, physical activity and the amount of salt the food you eat contains naturally. Early man obtained their required salt from a mostly meat diet. Only after man turned to agriculture did it became necessary to add salt to their diet because of the change in diet from a mostly meat to an increase of vegetables and cereals.
As stated, the amount of salt we will need in a long term survival situation will depend to a large extent on the food we eat. Cured meat has about 350 mg per serving, while fresh meat, poultry or fish have only about 75 mg per serving. Fresh vegetables have about 35 mg per serving, and fresh fruits have less than 10 mg per serving. As you can see, cured meats contain much more salt than fresh meat, the same is true for canned or dried vegetables. The natural sodium content of water, however, varies from 0 to over 500 mg per liter (a liter is about one quart), averaging 17 mg per liter.
In that we know that the recommended daily requirements for each person is 3.8 grams a day, this is the amount that should be cached for each person daily requirement. Calculated out this will equal a little over ¼ pound per month or a little over 3 pounds a year.
The problem of calculating how much salt to cache is the fact that there is another very important use for salt other than personal consumption. As a matter of fact the main use for salt in a long term survival situation will be in preserving foods. If you are salting, smoking, canning, pickling or making jerky you will need salt. The quantity of salt needed to preserve food will be much greater than that needed for individual consumption.
As a general rule, I would cache 25 pounds per year per person in the Group. I would want to cache enough salt to last at least two years. With a family of four, that would only be 200 pounds. This amount will allow more than enough salt for daily consumption, plus the salt for preserving food.
Since salt will not spoil it will be relatively easy to cache. The only problem will be water. The container will have to be water tight. Perhaps the best method will be to simply store the salt in 25 pound bags placed in either plastic or metal barrels with water tight lids. Another option would be to place a 25 pound bag of salt in sealable 5 gallon plastic buckets. Regardless of which container you use it should then be buried, preferably at your evacuation point.
Natural Sources of Salt:
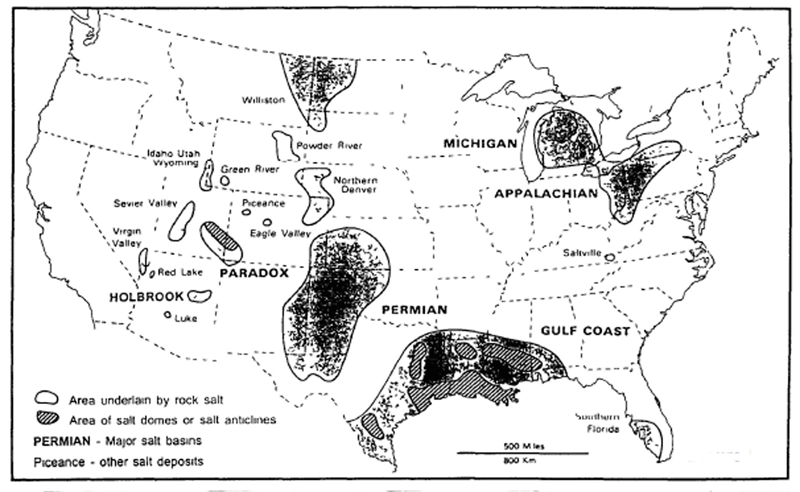
Early man soon discovered the use of salt in preserving meat. With this knowledge salt licks, salt springs and salt marshes became a necessity for survival. Out of necessity they had to remain in relatively close proximity to a source of salt. Although your Group has two or more years of salt stored, it will be a great asset in locating a natural source of salt close to your evacuation point. Luckily there are natural sources of salt throughout the U.S. However, in some areas these can be difficult to locate. In non-arid regions salt deposits can exist only below ground. Any deposits above ground would be dissolved by rain. A good indicator of underground salt deposit is the present of a salt crust on the ground. This salt crust will have the appearance of snow or frost. This salt crust will usually be found in a depression or low spot where water has stood for a period of time after a heavy rain. You can also find a salt deposit as it was done in ancient times. This is simply by following game trails to salt licks. These are also good indicators of underground salt deposits. In arid climates game trails may lead you to rock salt outcrops.
For survival purposes salt is usually found in three natural states: Rock Salt, Brine, and Evaporated Salt (also known as Solar Salt).
Rock Salt : Found in layers in the ground. In some arid regions it can be found as exposed rock outcrops. In its pure form it is colorless but is usually found colored by impurities. It is soft and easily broken into cubes. You can easily identify rock salt by the fact that it is readily soluble in water.
Brine : The term used for salty water from which salt can be produced. This can be from a salt spring or salt well, as well as sea water or a salt lake.
Evaporated Salt or Solar Salt : This is usually found as dried salt cakes on the shores of seas or salt lakes. It may also be found in Solar pans where salt crystallization occurs naturally in hot climates.
If your natural source of salt is rock salt then this can simply be broken up or dissolved as needed. If brine is your source of salt then the salt will have to be extracted by either boiling to evaporate or using Solar evaporation.
For boiling, the brine is simply placed in a large contained and boiled until only the salt remains. This method will be necessary when there is limited sunlight. With Solar evaporation you will require a large container which is then placed in direct sunlight. Of course, the larger the container the faster the process will work. Although this method will take much longer than boiling it, it is sometimes necessary when a fuel source is scarce.
If you are lucky enough to be in an area where there are salt pans then you simple have to harvest the salt cakes.
Summary:
As you can see a supply of salt in a long term survival situation does not need to be as large as most people think. Don't missunderstand, the more salt you can cache the better, but it will mostly be used in preserving food. Your Group should be able to survive without it being necessary to add additional salt to your diet. By this I mean other than what you use in preserving food. Of course, the exception will be if you are located in an arid area. In this case then you may have to add salt to your diet, however, in an arid climate you should be able to locate a natural supply of salt.